Optimal cutpoint for the methylation signal
Detection of the methylation signal with Methyl-IT
Robersy Sanchez
rus547@psu.edu
Mackenzie’s lab
Department of Biology.
Pennsylvania State University, University Park, PA 16802
rus547@psu.edu
Mackenzie’s lab
Department of Biology.
Pennsylvania State University, University Park, PA 16802
15 January 2024
Source:vignettes/Cutpoint_estimation_with_Methyl-IT.Rmd
Cutpoint_estimation_with_Methyl-IT.RmdAbstract
The discrimination of the methylation signal from the stochastic methylation background resultant from the standard (non-stressful) biological processes is a critical step for the genome-wide methylation analysis. Such a discrimination requires for the knowledge of the probability distribution of the information divergence of methylation levels and a proper evaluation of the classification performance of differentially methylated positions (DMPs) into two classes: DMPs from control and DMPs from treatment.
Motivation
The probability of extreme methylation changes occurring spontaneously in a control population by the stochastic fluctuations inherent to biochemical processes and DNA maintenance (1), requires the discrimination of this background variation from a biological treatment signal. The stochasticity of the the methylation process derives from the stochasticity inherent to biochemical processes (2, 3). There are fundamental physical reasons to acknowledge that biochemical processes are subject to noise and fluctuations (4, 5). So, regardless constant environment, statistically significant methylation changes can be found in control population with probability greater than zero and proportional to a Boltzmann factor (6).
Natural signals and those generated by human technology are not free of noise and, as mentioned above, the methylation signal is no exception. Only signal detection based approaches are designed to filter out the signal from the noise , in natural and in human generated signals.
The stochasticity of methylation regulatory machinery effects is presumed to reflect system heterogeneity; cells from the same tissue are not necessarily in the same state, and therefore, corresponding cytosine sites differ in their methylation status. Consequently, overall organismal response is conveyed as a statistical outcome that distinguishes the regulatory methylation signal from statistical background “noise”. Estimation of optimal cutoff value for the signal is an additional step to remove any remaining potential methylation background noise with probability \(0 ≤ \alpha ≤ 0.05\). We define as a methylation signal (DMP) each cytosine site with Hellinger Divergence values above the cutpoint (shown (7).
As a result, a differentially methylated position (DMP) as a cytosine position with high probability to be altered in methylation due to a treatment effect, distinct from spontaneous variation detected in the control population.
Note: This example was made with the MethylIT version 0.3.2 available at https://github.com/genomaths/MethylIT.
Data generation
For the current example on methylation analysis with Methyl-IT we will use simulated data. Read-count matrices of methylated and unmethylated cytosine are generated with MethylIT.utils function simulateCounts. A standard analysis of this dataset is given in the web page: Methylation analysis with Methyl-IT
library(MethylIT)
library(MethylIT.utils)
library(BiocParallel)
bmean <- function(alpha, beta) alpha/(alpha + beta)
alpha.ct <- 0.09
alpha.tt <- 0.2
# The number of cytosine sites to generate
sites = 50000
# Set a seed for pseudo-random number generation
set.seed(124)
control.nam <- c("C1", "C2", "C3")
treatment.nam <- c("T1", "T2", "T3")
# Reference group
ref0 = simulateCounts(num.samples = 4, sites = sites, alpha = alpha.ct,
beta = 0.5, size = 50, theta = 4.5,
sample.ids = c("R1", "R2", "R3", "R4"))
# Control group
ctrl = simulateCounts(num.samples = 3, sites = sites, alpha = alpha.ct,
beta = 0.5, size = 50, theta = 4.5,
sample.ids = control.nam)
# Treatment group
treat = simulateCounts(num.samples = 3, sites = sites, alpha = alpha.tt,
beta = 0.5, size = 50, theta = 4.5,
sample.ids = treatment.nam)
# Reference sample
ref = poolFromGRlist(ref0, stat = "mean", num.cores = 4L, verbose = FALSE)Methylation level divergences
Total variation distance and Hellinger divergence are computed with estimateDivergence function. The setting and.min.cov = TRUE in estimateDivergence will accomplish pairwise deletion of missing cytosine sites or sites no holding the filtering condition.
divs <- estimateDivergence(ref = ref,
indiv = c(ctrl, treat),
Bayesian = TRUE,
and.min.cov = FALSE,
num.cores = multicoreWorkers(),
percentile = 1, verbose = FALSE)To get some statistical description about the sample is useful. Here, empirical critical values for the probability distribution of \(H\) and \(TV\) can is obtained using quantile function from the R package stats.
critical.val <- do.call(rbind, lapply(divs, function(x) {
x <- x[x$hdiv > 0]
hd.95 = quantile(x$hdiv, 0.95)
tv.95 = quantile(abs(x$TV), 0.95)
return(c(tv = tv.95, hd = hd.95))
}))
critical.val## tv.95% hd.95%
## C1 0.6842105 66.76003
## C2 0.6800000 66.71971
## C3 0.6777456 65.98486
## T1 0.9397681 138.68200
## T2 0.9478351 141.72617
## T3 0.9466565 141.77236Estimation of potential DMPs with Methyl-IT
In Methyl-IT, DMP estimation requires for the knowledge of the probability distribution of the noise (plus signal), which is used as null hypothesis.
The best fitted distribution model can be estimated with function gofReport. Here, for illustration purposes, we will use specific estimations based on 2- and 3-parameter gamma distribution models directly using function nonlinearFitDist.
Potential DMPs estimated with 2-parameter gamma distribution model
nlms.g2p <- nonlinearFitDist(divs, column = 9L, verbose = FALSE,
num.cores = multicoreWorkers(),
dist.name = "Gamma2P")
# Potential DMPs from 'Gamma2P' model
pDMPs.g2p <- getPotentialDIMP(LR = divs, nlms = nlms.g2p, div.col = 9L,
tv.cut = 0.68, tv.col = 7, alpha = 0.05,
dist.name = "Gamma2P")Potential DMPs estimated with 3-parameter gamma distribution model
nlms.g3p <- nonlinearFitDist(divs, column = 9L,
verbose = FALSE,
num.cores = multicoreWorkers(),
dist.name = "Gamma3P")
# Potential DMPs from 'Gamma2P' model
pDMPs.g3p <- getPotentialDIMP(LR = divs, nlms = nlms.g3p, div.col = 9L,
tv.cut = 0.68, tv.col = 7, alpha = 0.05,
dist.name = "Gamma3P")Cutpoint estimation
As a result of the natural spontaneous variation, naturally occurring DMPs can be identified in samples from the control and treatment populations. Machine-learning algorithms implemented in Methyl-IT are applied to discriminate treatment-induced DMPs from those naturally generated.
The simple cutpoint estimation available in Methyl-IT is based on the application of Youden index (8). Although cutpoints are estimated for a single variable, the classification performance can be evaluated for several variables and applying different model classifiers.
In the current example, the column carrying TV (div.col = 7L) will be used to estimate the cutpoint. The column values will be expressed in terms of \(TV_d=|p_{tt}-p_{ct}|\). A minimum cutpoint value for TV derived from the minimum 95% quantile (tv.cut = 0.92) found in the treatment group will be applied (see Methylation analysis with Methyl-IT).
Next, a logistic model classifier will be fitted with the 60% (prop = 0.6) of the raw data (training set) and then the resting 40% of individual samples will be used to evaluate the model performance. The predictor variable included in the model are specified with function parameter column (for more detail see estimateCutPoint or type ?estimateCutPoint in R console).
Simple cutpoint estimation for Gamma2P model
Here, we use the results of modeling the distribution of the Hellinger divergence (HD) of methylation levels through a 2-parameter gamma probability distribution model. The critical values for \(HD_{\alpha = 0.05}^{CT_{G2P}}\) used to get potential DMPs were:
nams <- names(nlms.g2p)
crit <- unlist(lapply(nlms.g2p, function(x) qgamma(0.95, shape = x$Estimate[1],
scale = x$Estimate[2])))
names(crit) <- nams
crit## C1 C2 C3 T1 T2 T3
## 58.59151 57.99945 57.80987 112.39982 113.92337 114.48812As before the cutpoint is estimated based on ‘Youden Index’ (8). A PCA+LDA model classifier (classifier = “pca.lda”) is applied. That is, a principal component analysis (PCA) is applied on the original raw matrix of data and the four possible component (n.pc = 4) derived from the analysis are used in a further linear discriminant analysis (LDA). A scaling step is applied to the raw matrix of data before the application of the mentioned procedure (center = TRUE, scale = TRUE). Here, PCA will yield new orthogonal (non-correlated) variables, the principal components, which prevent any potential bias effect originated by any correlation or association of the original variables.
By using function estimateCutPoint, we can estimate the cutpoint, based on HD (div.col = 9L) or on \(TV_d\) (div.col = 7L):
# Cutpoint estimation for the FT approach using the ECDF critical value
cut.g2p = estimateCutPoint(LR = pDMPs.g2p,
simple = TRUE,
column = c(hdiv = TRUE, bay.TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier1 = "pca.lda", n.pc = 4,
control.names = control.nam,
treatment.names = treatment.nam,
center = TRUE, scale = TRUE,
clas.perf = TRUE, prop = 0.6,
div.col = 9L)
cut.g2p## Cutpoint estimation with 'Youden Index'
## Simple cutpoint estimation
## Cutpoint = 114.22
##
## Cytosine sites from treatment have divergence values >= 114.22
##
## The accessible objects in the output list are:
## Length Class Mode
## cutpoint 1 -none- numeric
## testSetPerformance 6 confusionMatrix list
## testSetModel.FDR 1 -none- numeric
## model 5 pcaLDA list
## modelConfMatrix 6 confusionMatrix list
## initModel 1 -none- character
## postProbCut 1 -none- logical
## postCut 1 -none- logical
## classifier 1 -none- character
## statistic 1 -none- logical
## optStatVal 1 -none- logical
## cutpData 1 -none- logical
## initModelConfMatrix 6 confusionMatrix listAs indicated above, the model classifier performance and its corresponding false discovery rate can be retrieved as:
cut.g2p$testSetPerformance## Confusion Matrix and Statistics
##
## Reference
## Prediction CT TT
## CT 319 0
## TT 0 3544
##
## Accuracy : 1
## 95% CI : (0.999, 1)
## No Information Rate : 0.9174
## P-Value [Acc > NIR] : < 2.2e-16
##
## Kappa : 1
##
## Mcnemar's Test P-Value : NA
##
## Sensitivity : 1.0000
## Specificity : 1.0000
## Pos Pred Value : 1.0000
## Neg Pred Value : 1.0000
## Prevalence : 0.9174
## Detection Rate : 0.9174
## Detection Prevalence : 0.9174
## Balanced Accuracy : 1.0000
##
## 'Positive' Class : TT
##
cut.g2p$testSetModel.FDR## [1] 0Here, DMP classification is modeled with PCA+QDA classifier (classifier = “pca.lda”). That is, principal component analysis (PCA) is applied on the original raw matrix of data and the four possible component (n.pc = 4) are used in a further linear discriminant analysis (LDA). A scaling step is applied to the raw matrix of data before the application of the mentioned procedure (center = TRUE, scale = TRUE). Next, a different model classifier can be applied to model the classification derived from the previous cutpoint estimation.
Simple cutpoint estimation for Gamma3P model
The same analyses for the cutpoint estimation can be performed for 3P gamma model
# Cutpoint estimation for the FT approach using the ECDF critical value
cut.g3p = estimateCutPoint(LR = pDMPs.g3p, simple = TRUE,
column = c(hdiv = TRUE, bay.TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier1 = "pca.lda", n.pc = 4,
control.names = control.nam,
treatment.names = treatment.nam,
center = TRUE, scale = TRUE,
clas.perf = TRUE, prop = 0.6,
div.col = 9L)
cut.g3p## Cutpoint estimation with 'Youden Index'
## Simple cutpoint estimation
## Cutpoint = 115.24
##
## Cytosine sites from treatment have divergence values >= 115.24
##
## The accessible objects in the output list are:
## Length Class Mode
## cutpoint 1 -none- numeric
## testSetPerformance 6 confusionMatrix list
## testSetModel.FDR 1 -none- numeric
## model 5 pcaLDA list
## modelConfMatrix 6 confusionMatrix list
## initModel 1 -none- character
## postProbCut 1 -none- logical
## postCut 1 -none- logical
## classifier 1 -none- character
## statistic 1 -none- logical
## optStatVal 1 -none- logical
## cutpData 1 -none- logical
## initModelConfMatrix 6 confusionMatrix listAs indicated above, the model classifier performance and its corresponding false discovery rate can be retrieved as:
cut.g3p$testSetPerformance## Confusion Matrix and Statistics
##
## Reference
## Prediction CT TT
## CT 309 0
## TT 0 3481
##
## Accuracy : 1
## 95% CI : (0.999, 1)
## No Information Rate : 0.9185
## P-Value [Acc > NIR] : < 2.2e-16
##
## Kappa : 1
##
## Mcnemar's Test P-Value : NA
##
## Sensitivity : 1.0000
## Specificity : 1.0000
## Pos Pred Value : 1.0000
## Neg Pred Value : 1.0000
## Prevalence : 0.9185
## Detection Rate : 0.9185
## Detection Prevalence : 0.9185
## Balanced Accuracy : 1.0000
##
## 'Positive' Class : TT
##
cut.g3p$testSetModel.FDR## [1] 0DMP prediction based on classification models
The model obtained in the previous step can be used for further prediction with function predict. For example, we would take a random sample and run:
set.seed(1)
randsampl <- unlist(pDMPs.g3p)
randsampl <- randsampl[sample.int(length(randsampl), 10)]
pred <- predict(cut.g3p$model, newdata = randsampl)
pred## $class
## [1] CT TT TT TT TT TT TT TT TT TT
## Levels: CT TT
##
## $posterior
## CT TT
## [1,] 1.000000e+00 9.949362e-09
## [2,] 1.323743e-46 1.000000e+00
## [3,] 1.435707e-45 1.000000e+00
## [4,] 2.648143e-46 1.000000e+00
## [5,] 7.965328e-48 1.000000e+00
## [6,] 1.108810e-46 1.000000e+00
## [7,] 1.260233e-46 1.000000e+00
## [8,] 8.773698e-47 1.000000e+00
## [9,] 4.528686e-47 1.000000e+00
## [10,] 3.130172e-54 1.000000e+00
##
## $x
## LD1
## [1,] -7.4843076
## [2,] 1.1181324
## [3,] 0.9528444
## [4,] 1.0700533
## [5,] 1.3130110
## [6,] 1.1304175
## [7,] 1.1215415
## [8,] 1.1466507
## [9,] 1.1925061
## [10,] 2.3357223The variable pred$posterior provides the posterior classification probabilities that a DMP could belong to control (CT) or to treatment (TT) group. The variable ‘x’ provides the cytosine methylation changes in terms of its values in the linear discriminant function LD1. Notice that, for each row, the sum of posterior probabilities is equal 1. By default, individuals with TT posterior probabilities greater than 0.5 are predicted to belong to the treatment class. For example:
classfiction = rep("CT", 10)
classfiction[pred$posterior[, 2] > 0.5] <- "TT"
classfiction## [1] "CT" "TT" "TT" "TT" "TT" "TT" "TT" "TT" "TT" "TT"We can be more strict increasing the posterior classification probability cutoff
classfiction = rep("CT", 10)
classfiction[pred$posterior[, 2] > 0.7] <- "TT"
classfiction## [1] "CT" "TT" "TT" "TT" "TT" "TT" "TT" "TT" "TT" "TT"The posterior classification probability cutoff can be controlled with parameter post.cut from estimateCutPoint function (default: \(post.cut=0.5\)).
Machine-learning (ML) based approach to search for an optimal cutpoint
In the next example the cutpoint estimation for the Hellinger divergence of methylation levels (div.col = 9L) is accomplished. Function estimateCutPoint can be used to search for a cutpoint as well. Two model classifiers can be used. classifiers1 will be used to estimate the posterior classification probabilities of DMP into those from control and those from treatment. These probabilities are then used to estimate the cutpoint in the range of values from, say, 0.5 to 0.8. Next, a classifier2 will be used to evaluate the classification performance. In this case, the search for an optimal cutpoint is accomplished maximizing the accuracy (stat = 0) of classifier2.
ML cutpoint estimation for potential DMPs based on Gamma2P model
The ML search for an optimal cutpoint is accomplished in the set of potential DMPs, which were identified using a Gamma2P probability distribution model as null hypothesis.
cut.g2p = estimateCutPoint(LR = pDMPs.g2p, simple = FALSE,
column = c(hdiv = TRUE, bay.TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier1 = "pca.lda",
classifier2 = "pca.qda", stat = 0,
control.names = control.nam,
treatment.names = treatment.nam,
cut.values = seq(45, 114, 1), post.cut = 0.5,
prop = 0.6, center = TRUE, scale = TRUE,
n.pc = 4, div.col = 9L)
cut.g2p## Cutpoint estimation with 'pca.lda' classifier
## Cutpoint search performed using model posterior probabilities
##
## Posterior probability used to get the cutpoint = 0.5
## Cytosine sites with treatment PostProbCut >= 0.5 have a
## divergence value >= 112.4245
##
## Optimized statistic: Accuracy = 1
## Cutpoint = 112.42
##
## Model classifier 'pca.qda'
##
## The accessible objects in the output list are:
## Length Class Mode
## cutpoint 1 -none- numeric
## testSetPerformance 6 confusionMatrix list
## testSetModel.FDR 1 -none- numeric
## model 5 pcaQDA list
## modelConfMatrix 6 confusionMatrix list
## initModel 1 -none- character
## postProbCut 1 -none- numeric
## postCut 1 -none- numeric
## classifier 1 -none- character
## statistic 1 -none- character
## optStatVal 1 -none- numeric
## cutpData 1 -none- logicalModel performance in the test dataset is:
cut.g2p$testSetPerformance## Confusion Matrix and Statistics
##
## Reference
## Prediction CT TT
## CT 1274 0
## TT 0 3580
##
## Accuracy : 1
## 95% CI : (0.9992, 1)
## No Information Rate : 0.7375
## P-Value [Acc > NIR] : < 2.2e-16
##
## Kappa : 1
##
## Mcnemar's Test P-Value : NA
##
## Sensitivity : 1.0000
## Specificity : 1.0000
## Pos Pred Value : 1.0000
## Neg Pred Value : 1.0000
## Prevalence : 0.7375
## Detection Rate : 0.7375
## Detection Prevalence : 0.7375
## Balanced Accuracy : 1.0000
##
## 'Positive' Class : TT
## Model performance in in the whole dataset is:
cut.g2p$modelConfMatrix## Confusion Matrix and Statistics
##
## Reference
## Prediction CT TT
## CT 3184 0
## TT 0 8948
##
## Accuracy : 1
## 95% CI : (0.9997, 1)
## No Information Rate : 0.7376
## P-Value [Acc > NIR] : < 2.2e-16
##
## Kappa : 1
##
## Mcnemar's Test P-Value : NA
##
## Sensitivity : 1.0000
## Specificity : 1.0000
## Pos Pred Value : 1.0000
## Neg Pred Value : 1.0000
## Prevalence : 0.7376
## Detection Rate : 0.7376
## Detection Prevalence : 0.7376
## Balanced Accuracy : 1.0000
##
## 'Positive' Class : TT
## The False discovery rate is:
cut.g2p$testSetModel.FDR## [1] 0The model classifier PCA+LDA has enough discriminatory power to discriminate control DMP from those induced by the treatment for HD values \(112.4247 \le HD\).
The probabilities \(P(HD \le 122.43)\) to observe a cytosine site with \(HD \le 112.4247\) on each individual is:
nams <- names(nlms.g2p)
crit <- unlist(lapply(nlms.g2p,
function(x)
pgamma(cut.g2p$cutpoint, shape = x$Estimate[1],
scale = x$Estimate[2])))
names(crit) <- nams
crit## C1 C2 C3 T1 T2 T3
## 0.9964705 0.9966560 0.9967314 0.9500280 0.9483024 0.9476607In other words, most of the methylation changes are not (and should not be) considered DMPs. Notice that although the same HD value could be found in the same differentially methylated cytosine site in control and treatment, if the probabilities distributions of the information divergences (null hypotheses) from control and treatment are different, then these DMPs can be distinguished.
ML cutpoint estimation for potential DMPs based on Gamma3P model
Likewise, for the 3-parameter gamma model we can search for an optimal cutpoint:
cut.g3p = estimateCutPoint(LR = pDMPs.g3p, simple = FALSE,
column = c(hdiv = TRUE, TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier1 = "pca.lda",
classifier2 = "pca.qda", stat = 0,
control.names = control.nam,
treatment.names = treatment.nam,
cut.values = seq(45, 114, 1), post.cut = 0.5,
clas.perf = TRUE, prop = 0.6,
center = TRUE, scale = TRUE,
n.pc = 4, div.col = 9L)
cut.g3p## Cutpoint estimation with 'pca.lda' classifier
## Cutpoint search performed using model posterior probabilities
##
## Posterior probability used to get the cutpoint = 0.5
## Cytosine sites with treatment PostProbCut >= 0.5 have a
## divergence value >= 113.5828
##
## Optimized statistic: Accuracy = 1
## Cutpoint = 113.58
##
## Model classifier 'pca.qda'
##
## The accessible objects in the output list are:
## Length Class Mode
## cutpoint 1 -none- numeric
## testSetPerformance 6 confusionMatrix list
## testSetModel.FDR 1 -none- numeric
## model 5 pcaQDA list
## modelConfMatrix 6 confusionMatrix list
## initModel 1 -none- character
## postProbCut 1 -none- numeric
## postCut 1 -none- numeric
## classifier 1 -none- character
## statistic 1 -none- character
## optStatVal 1 -none- numeric
## cutpData 1 -none- logicalDMPs are identified with function selectDIMP
g2p.dmps <- selectDIMP(pDMPs.g2p, div.col = 9L, cutpoint = cut.g2p$cutpoint)
g3p.dmps <- selectDIMP(pDMPs.g3p, div.col = 9L, cutpoint = cut.g3p$cutpoint)DMPs estimated with Fisher’s exact Test (FT)
For comparison purposes DMPs are estimated with Fisher’s exact test as well.
ft. = FisherTest(LR = divs, tv.cut = 0.68,
pAdjustMethod = "BH",
pvalCutOff = 0.05,
num.cores = multicoreWorkers(),
verbose = FALSE, saveAll = FALSE)
ft.dmps <- getPotentialDIMP(LR = ft., div.col = 9L, dist.name = "None",
tv.cut = 0.68, tv.col = 7, alpha = 0.05)Classification performance of DMP classification
After the cutpoint application to select DMPs, a Monte Carlo (bootstrap) analysis reporting several classifier performance indicators can be accomplished by using function evaluateDIMPclass and its settings output = “mc.val” and num.boot.
Classification performance for DMPs based on Gamma2P model
g2p.class = evaluateDIMPclass(LR = g2p.dmps, control.names = control.nam,
treatment.names = treatment.nam,
column = c(hdiv = TRUE, TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier = "pca.qda", n.pc = 4,
center = TRUE, scale = TRUE, num.boot = 300,
output = "all", prop = 0.6
)
g2p.class$mc.val## Accuracy Kappa AccuracyLower AccuracyUpper AccuracyNull
## Min. :1 Min. :1 Min. :0.9991 Min. :1 Min. :0.9139
## 1st Qu.:1 1st Qu.:1 1st Qu.:0.9991 1st Qu.:1 1st Qu.:0.9139
## Median :1 Median :1 Median :0.9991 Median :1 Median :0.9139
## Mean :1 Mean :1 Mean :0.9991 Mean :1 Mean :0.9139
## 3rd Qu.:1 3rd Qu.:1 3rd Qu.:0.9991 3rd Qu.:1 3rd Qu.:0.9139
## Max. :1 Max. :1 Max. :0.9991 Max. :1 Max. :0.9139
##
## AccuracyPValue McnemarPValue Sensitivity Specificity Pos Pred Value
## Min. :9.096e-154 Min. : NA Min. :1 Min. :1 Min. :1
## 1st Qu.:9.096e-154 1st Qu.: NA 1st Qu.:1 1st Qu.:1 1st Qu.:1
## Median :9.096e-154 Median : NA Median :1 Median :1 Median :1
## Mean :9.096e-154 Mean :NaN Mean :1 Mean :1 Mean :1
## 3rd Qu.:9.096e-154 3rd Qu.: NA 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1
## Max. :9.096e-154 Max. : NA Max. :1 Max. :1 Max. :1
## NA's :300
## Neg Pred Value Precision Recall F1 Prevalence
## Min. :1 Min. :1 Min. :1 Min. :1 Min. :0.9139
## 1st Qu.:1 1st Qu.:1 1st Qu.:1 1st Qu.:1 1st Qu.:0.9139
## Median :1 Median :1 Median :1 Median :1 Median :0.9139
## Mean :1 Mean :1 Mean :1 Mean :1 Mean :0.9139
## 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1 3rd Qu.:0.9139
## Max. :1 Max. :1 Max. :1 Max. :1 Max. :0.9139
##
## Detection Rate Detection Prevalence Balanced Accuracy
## Min. :0.9139 Min. :0.9139 Min. :1
## 1st Qu.:0.9139 1st Qu.:0.9139 1st Qu.:1
## Median :0.9139 Median :0.9139 Median :1
## Mean :0.9139 Mean :0.9139 Mean :1
## 3rd Qu.:0.9139 3rd Qu.:0.9139 3rd Qu.:1
## Max. :0.9139 Max. :0.9139 Max. :1
## Classification performance for DMPs based on Gamma3P model
Likewise the evaluation of the DMP classification performance can be accomplished for DMPs estimated based on the \('Gamma3P'\) model:
g3p.class = evaluateDIMPclass(LR = g3p.dmps, control.names = control.nam,
treatment.names = treatment.nam,
column = c(hdiv = TRUE, TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier = "pca.qda", n.pc = 4,
center = TRUE, scale = TRUE, num.boot = 300,
output = "all", prop = 0.6
)
g3p.class$mc.val## Accuracy Kappa AccuracyLower AccuracyUpper AccuracyNull
## Min. :1 Min. :1 Min. :0.999 Min. :1 Min. :0.914
## 1st Qu.:1 1st Qu.:1 1st Qu.:0.999 1st Qu.:1 1st Qu.:0.914
## Median :1 Median :1 Median :0.999 Median :1 Median :0.914
## Mean :1 Mean :1 Mean :0.999 Mean :1 Mean :0.914
## 3rd Qu.:1 3rd Qu.:1 3rd Qu.:0.999 3rd Qu.:1 3rd Qu.:0.914
## Max. :1 Max. :1 Max. :0.999 Max. :1 Max. :0.914
##
## AccuracyPValue McnemarPValue Sensitivity Specificity Pos Pred Value
## Min. :1.387e-150 Min. : NA Min. :1 Min. :1 Min. :1
## 1st Qu.:1.387e-150 1st Qu.: NA 1st Qu.:1 1st Qu.:1 1st Qu.:1
## Median :1.387e-150 Median : NA Median :1 Median :1 Median :1
## Mean :1.387e-150 Mean :NaN Mean :1 Mean :1 Mean :1
## 3rd Qu.:1.387e-150 3rd Qu.: NA 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1
## Max. :1.387e-150 Max. : NA Max. :1 Max. :1 Max. :1
## NA's :300
## Neg Pred Value Precision Recall F1 Prevalence
## Min. :1 Min. :1 Min. :1 Min. :1 Min. :0.914
## 1st Qu.:1 1st Qu.:1 1st Qu.:1 1st Qu.:1 1st Qu.:0.914
## Median :1 Median :1 Median :1 Median :1 Median :0.914
## Mean :1 Mean :1 Mean :1 Mean :1 Mean :0.914
## 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1 3rd Qu.:0.914
## Max. :1 Max. :1 Max. :1 Max. :1 Max. :0.914
##
## Detection Rate Detection Prevalence Balanced Accuracy
## Min. :0.914 Min. :0.914 Min. :1
## 1st Qu.:0.914 1st Qu.:0.914 1st Qu.:1
## Median :0.914 Median :0.914 Median :1
## Mean :0.914 Mean :0.914 Mean :1
## 3rd Qu.:0.914 3rd Qu.:0.914 3rd Qu.:1
## Max. :0.914 Max. :0.914 Max. :1
## We do not have evidence to support statistical differences between the classification performances estimated for ‘Gamma2P’ and ‘Gamma3P’ probability distribution models. Hence, in this case we select the model that yield the lowest cutpoint
Classification performance for DMPs based on Fisher’s exact test
Classification performance results obtained with Monte Carlos sampling for the \(Gamma2P\) and \(Gamma3P\) models are quite different from those obtained with FT:
ft.class = evaluateDIMPclass(LR = ft.dmps, control.names = control.nam,
treatment.names = treatment.nam,
column = c(hdiv = TRUE, TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier = "pca.lda", n.pc = 4,
center = TRUE, scale = TRUE, num.boot = 300,
output = "all", prop = 0.6
)
ft.class$mc.val## Accuracy Kappa AccuracyLower AccuracyUpper
## Min. :0.8086 Min. :-0.002712 Min. :0.8011 Min. :0.8158
## 1st Qu.:0.8103 1st Qu.: 0.004770 1st Qu.:0.8030 1st Qu.:0.8176
## Median :0.8110 Median : 0.007210 Median :0.8036 Median :0.8182
## Mean :0.8109 Mean : 0.007260 Mean :0.8036 Mean :0.8181
## 3rd Qu.:0.8115 3rd Qu.: 0.009902 3rd Qu.:0.8041 3rd Qu.:0.8187
## Max. :0.8138 Max. : 0.017490 Max. :0.8065 Max. :0.8210
## AccuracyNull AccuracyPValue McnemarPValue Sensitivity
## Min. :0.8188 Min. :0.9168 Min. :0 Min. :0.9834
## 1st Qu.:0.8188 1st Qu.:0.9781 1st Qu.:0 1st Qu.:0.9857
## Median :0.8188 Median :0.9847 Median :0 Median :0.9864
## Mean :0.8188 Mean :0.9828 Mean :0 Mean :0.9863
## 3rd Qu.:0.8188 3rd Qu.:0.9902 3rd Qu.:0 3rd Qu.:0.9869
## Max. :0.8188 Max. :0.9976 Max. :0 Max. :0.9890
## Specificity Pos Pred Value Neg Pred Value Precision
## Min. :0.01233 Min. :0.8186 Min. :0.1636 Min. :0.8186
## 1st Qu.:0.01677 1st Qu.:0.8193 1st Qu.:0.2117 1st Qu.:0.8193
## Median :0.01824 Median :0.8195 Median :0.2291 Median :0.8195
## Mean :0.01838 Mean :0.8195 Mean :0.2286 Mean :0.8195
## 3rd Qu.:0.01972 3rd Qu.:0.8198 3rd Qu.:0.2458 3rd Qu.:0.8198
## Max. :0.02465 Max. :0.8205 Max. :0.3082 Max. :0.8205
## Recall F1 Prevalence Detection Rate
## Min. :0.9834 Min. :0.8938 Min. :0.8188 Min. :0.8053
## 1st Qu.:0.9857 1st Qu.:0.8949 1st Qu.:0.8188 1st Qu.:0.8071
## Median :0.9864 Median :0.8952 Median :0.8188 Median :0.8077
## Mean :0.9863 Mean :0.8952 Mean :0.8188 Mean :0.8076
## 3rd Qu.:0.9869 3rd Qu.:0.8956 3rd Qu.:0.8188 3rd Qu.:0.8081
## Max. :0.9890 Max. :0.8969 Max. :0.8188 Max. :0.8098
## Detection Prevalence Balanced Accuracy
## Min. :0.9828 Min. :0.4991
## 1st Qu.:0.9849 1st Qu.:0.5015
## Median :0.9854 Median :0.5023
## Mean :0.9854 Mean :0.5023
## 3rd Qu.:0.9860 3rd Qu.:0.5032
## Max. :0.9879 Max. :0.5056A quite different story is found when information on the probability distribution of noise (null hypothesis) is added to the classifier:
ft.g2p.dmps <- getPotentialDIMP(LR = ft., nlms = nlms.g2p, div.col = 9L,
tv.cut = 0.68, tv.col = 7, alpha = 0.05,
dist.name = "Gamma2P")
ft.g2p.class = evaluateDIMPclass(LR = ft.g2p.dmps, control.names = control.nam,
treatment.names = treatment.nam,
column = c(hdiv = TRUE, TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier = "pca.lda", n.pc = 4,
center = TRUE, scale = TRUE,
num.boot = 300,
num.cores = multicoreWorkers(),
output = "all",
prop = 0.6
)
ft.g2p.class$mc.val## Accuracy Kappa AccuracyLower AccuracyUpper AccuracyNull
## Min. :1 Min. :1 Min. :0.9992 Min. :1 Min. :0.7375
## 1st Qu.:1 1st Qu.:1 1st Qu.:0.9992 1st Qu.:1 1st Qu.:0.7375
## Median :1 Median :1 Median :0.9992 Median :1 Median :0.7375
## Mean :1 Mean :1 Mean :0.9992 Mean :1 Mean :0.7375
## 3rd Qu.:1 3rd Qu.:1 3rd Qu.:0.9992 3rd Qu.:1 3rd Qu.:0.7375
## Max. :1 Max. :1 Max. :0.9992 Max. :1 Max. :0.7375
##
## AccuracyPValue McnemarPValue Sensitivity Specificity Pos Pred Value
## Min. :0 Min. : NA Min. :1 Min. :1 Min. :1
## 1st Qu.:0 1st Qu.: NA 1st Qu.:1 1st Qu.:1 1st Qu.:1
## Median :0 Median : NA Median :1 Median :1 Median :1
## Mean :0 Mean :NaN Mean :1 Mean :1 Mean :1
## 3rd Qu.:0 3rd Qu.: NA 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1
## Max. :0 Max. : NA Max. :1 Max. :1 Max. :1
## NA's :300
## Neg Pred Value Precision Recall F1 Prevalence
## Min. :1 Min. :1 Min. :1 Min. :1 Min. :0.7375
## 1st Qu.:1 1st Qu.:1 1st Qu.:1 1st Qu.:1 1st Qu.:0.7375
## Median :1 Median :1 Median :1 Median :1 Median :0.7375
## Mean :1 Mean :1 Mean :1 Mean :1 Mean :0.7375
## 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1 3rd Qu.:0.7375
## Max. :1 Max. :1 Max. :1 Max. :1 Max. :0.7375
##
## Detection Rate Detection Prevalence Balanced Accuracy
## Min. :0.7375 Min. :0.7375 Min. :1
## 1st Qu.:0.7375 1st Qu.:0.7375 1st Qu.:1
## Median :0.7375 Median :0.7375 Median :1
## Mean :0.7375 Mean :0.7375 Mean :1
## 3rd Qu.:0.7375 3rd Qu.:0.7375 3rd Qu.:1
## Max. :0.7375 Max. :0.7375 Max. :1
## Now, we add additional information about the optimal cutpoint
ft.g2p_cutp.dmps <- selectDIMP(ft.g2p.dmps, div.col = 9L,
cutpoint = cut.g2p$cutpoint)
ft.g2p_cut.class = evaluateDIMPclass(LR = ft.g2p_cutp.dmps,
control.names = control.nam,
treatment.names = treatment.nam,
column = c(hdiv = TRUE, TV = TRUE,
wprob = TRUE, pos = TRUE),
classifier = "pca.lda", n.pc = 4,
center = TRUE, scale = TRUE, num.boot = 300,
output = "all", prop = 0.6
)
ft.g2p_cut.class$mc.val## Accuracy Kappa AccuracyLower AccuracyUpper AccuracyNull
## Min. :1 Min. :1 Min. :0.9991 Min. :1 Min. :0.9139
## 1st Qu.:1 1st Qu.:1 1st Qu.:0.9991 1st Qu.:1 1st Qu.:0.9139
## Median :1 Median :1 Median :0.9991 Median :1 Median :0.9139
## Mean :1 Mean :1 Mean :0.9991 Mean :1 Mean :0.9139
## 3rd Qu.:1 3rd Qu.:1 3rd Qu.:0.9991 3rd Qu.:1 3rd Qu.:0.9139
## Max. :1 Max. :1 Max. :0.9991 Max. :1 Max. :0.9139
##
## AccuracyPValue McnemarPValue Sensitivity Specificity Pos Pred Value
## Min. :9.096e-154 Min. : NA Min. :1 Min. :1 Min. :1
## 1st Qu.:9.096e-154 1st Qu.: NA 1st Qu.:1 1st Qu.:1 1st Qu.:1
## Median :9.096e-154 Median : NA Median :1 Median :1 Median :1
## Mean :9.096e-154 Mean :NaN Mean :1 Mean :1 Mean :1
## 3rd Qu.:9.096e-154 3rd Qu.: NA 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1
## Max. :9.096e-154 Max. : NA Max. :1 Max. :1 Max. :1
## NA's :300
## Neg Pred Value Precision Recall F1 Prevalence
## Min. :1 Min. :1 Min. :1 Min. :1 Min. :0.9139
## 1st Qu.:1 1st Qu.:1 1st Qu.:1 1st Qu.:1 1st Qu.:0.9139
## Median :1 Median :1 Median :1 Median :1 Median :0.9139
## Mean :1 Mean :1 Mean :1 Mean :1 Mean :0.9139
## 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1 3rd Qu.:1 3rd Qu.:0.9139
## Max. :1 Max. :1 Max. :1 Max. :1 Max. :0.9139
##
## Detection Rate Detection Prevalence Balanced Accuracy
## Min. :0.9139 Min. :0.9139 Min. :1
## 1st Qu.:0.9139 1st Qu.:0.9139 1st Qu.:1
## Median :0.9139 Median :0.9139 Median :1
## Mean :0.9139 Mean :0.9139 Mean :1
## 3rd Qu.:0.9139 3rd Qu.:0.9139 3rd Qu.:1
## Max. :0.9139 Max. :0.9139 Max. :1
## In other words, information on the probability distributions of the natural spontaneous methylation variation in the control and treatment population are essential to discriminate the background noise from the treatment induced signal.
Graphics of DMP classification performance
DMP count data:
dt <- t(rbind(G2P = sapply(g2p.dmps, length),
G3P = sapply(g3p.dmps, length),
FT = sapply(ft.dmps, length),
FT.G2P = sapply(ft.g2p.dmps, length),
FT.SD = sapply(ft.g2p_cutp.dmps, length)
))
dt## G2P G3P FT FT.G2P FT.SD
## C1 255 251 1730 1055 255
## C2 306 298 1682 1070 306
## C3 281 276 1657 1059 281
## T1 2925 2870 7589 2926 2925
## T2 3032 2961 7646 3032 3032
## T3 2990 2935 7679 2990 2990The comparison between the approaches FT.G2P and FT.SD (full signal detection on FT output) tells us that only 255, 306, and 281 cytosine sites detected with FT in the control samples C1, C2, and C3, respectively, carry methylation signals comparable (in magnitude) to those signals induced by the treatment.
Classification performance data:
df <- data.frame(method = c("FT", "FT.G2P", "FT.SD", "G2P", "G3P"),
rbind(c(colMeans(ft.class$boots)[c(1, 8:11, 18)],
FDR = ft.class$con.mat$FDR),
c(colMeans(ft.g2p.class$boots)[c(1, 8:11, 18)],
FDR = ft.g2p.class$con.mat$FDR),
c(colMeans(ft.g2p_cut.class$boots)[c(1, 8:11, 18)],
FDR = ft.g2p_cut.class$con.mat$FDR),
c(colMeans(g2p.class$boots)[c(1, 8:11, 18)],
FDR = g2p.class$con.mat$FDR),
c(colMeans(g3p.class$boots)[c(1, 8:11, 18)],
FDR = g3p.class$con.mat$FDR)
))Graphics:
color <- c("darkgreen", "#147714FF", "#3D9F3DFF", "#66C666FF", "#90EE90FF")
dt <- data.frame(dt, sample = names(g2p.dmps))
## ------------------------- DMP count graphic ---------------------------------
par(family = "serif", lwd = 0.1, cex = 1, mar = c(2,5,2,2), mfcol = c(1, 2))
barplot(cbind(FT, FT.G2P, FT.SD, G2P, G3P) ~ sample,
panel.first={points(0, 0, pch=16, cex=1e6, col="grey95")
grid(col="white", lty = 1, lwd = 1)},
data = dt, beside = TRUE, legend.text = TRUE, las = 1, lwd = 0.05,
yaxt = "n", cex.names = 1.4, font = 3, xlab = "", col = color,
args.legend = list(x=10, y=6000, text.font = 2, box.lwd = 0,
horiz = FALSE, adj = 0, xjust = 0.65, yjust = 0.8,
bty = "n", cex = 1.2, x.intersp = 0.2, inset = -1,
ncol = 1, fill = color))
axis(2, hadj = 0.9, las = 2, lwd = 0.4, tck = -0.02, cex.axis = 1.2, font = 2,
line = -0.2)
mtext(side = 2, text = "DMP count", line = 3, cex = 1.4, font = 3)
## ------------------ DMP classifiction performance graphic -------------------
color <- c("mediumblue", "#0000FFFF", "#3949F6FF", "#566CF2FF", "#7390EEFF",
"#90B3EAFF", "#ADD8E6FF")
labs <- df$method
par(family = "serif", lwd = 0.1, cex = 1, mar = c(4,2,2,10))
x <- barplot(cbind(Accuracy, Sensitivity, Specificity, Pos.Pred.Value,
Neg.Pred.Value, Balanced.Accuracy, FDR) ~ method,
panel.first = {points(0, 0, pch=16, cex=1e6, col="grey95")
grid(col="white", lty = 1, lwd = 1)},
data = df, beside = TRUE, legend.text = TRUE, las = 1, lwd = 0.1,
yaxt = "n", cex.names = 1.4, font = 3, xlab = "", col = color,
ylim = c(0,1),
args.legend = list(x = 52, y = 1., text.font = 2, box.lwd = 0,
horiz = FALSE, adj = 0, xjust = 0.65, yjust = 0.8,
bty = "n", cex = 1.2, x.intersp = 0.2, inset = -1,
ncol = 1, fill = color))
axis(2, hadj = 0.8, las = 2, lwd = 0.4, tck = -0.02, cex.axis = 1.2, font = 2,
line = -0.4)
mtext(side = 2, text = "Performance value", line = 2, cex = 1.4, font = 3)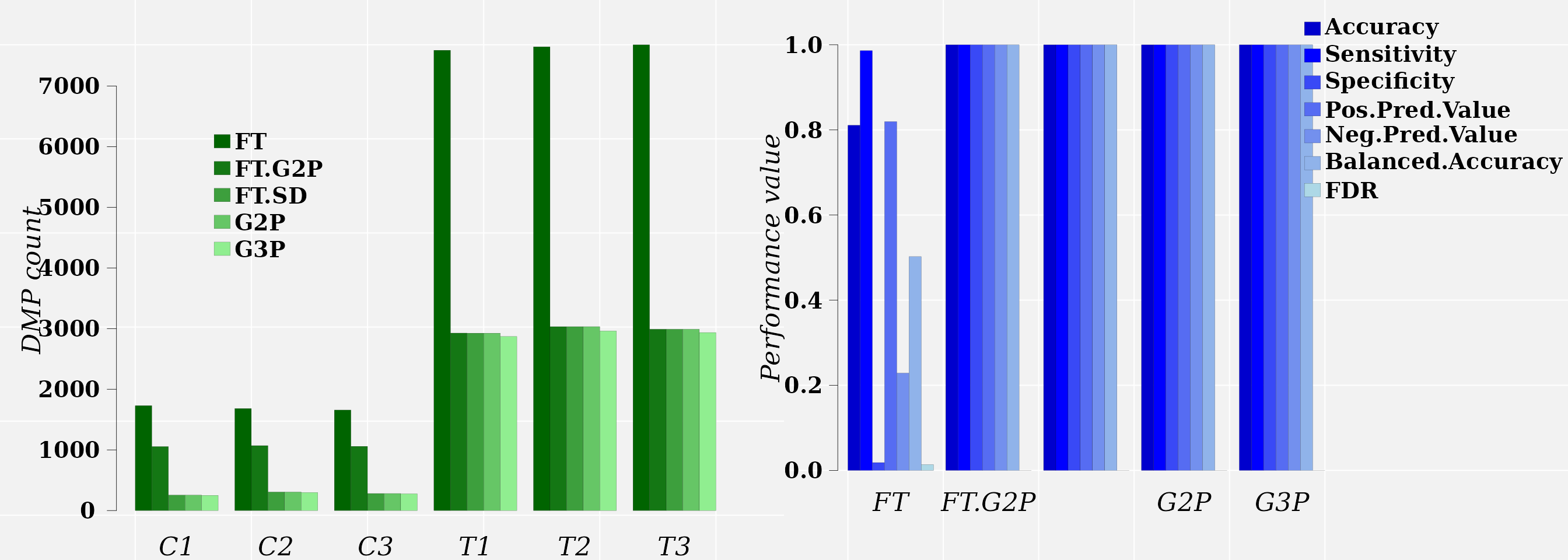
FT.G2P and FT.SD approaches lead to excellent classification performances on this data set. At this point, we can appeal the parsimony principle, follows from Occam’s razor that states “among competing hypotheses, the hypothesis with the fewest assumptions should be selected. In other words, results indicates that the signal-detection and machine-learning approach is sufficient (7, 9).
Conclusions
A proper discrimination of the methylation signal from the stochastic methylation background requires for the knowledge of probability distributions of the methylation signal from control and treatment population. Such a knowledge permits a suitable estimation of the cutoff value to discriminate the methylation signal induced by the treatment from the stochastic methylation background detected in the control group.
It does not matter how significant a differentially methylation event for a given cytosine position would be (after the application of some statistical test), but on how big the probability to be observed in the control group is. In simple words, if for a given DMP the probability of to be observed in the control is big enough, then such a DMP did not result from a treatment effect.
A suitable evaluation on how much the mentioned probability can be big enough derives by estimating an optimal cutpoint. But a classification into two groups results from the cutpoint estimation and the problem on the estimation of such a cutpoint is equivalent to find a discriminatory function (as set by Fisher, (10, 11)). Cases with function values below some cutoff are classified in one group, while values above the cutoff are put in the other group.
MethylIT function estimateCutPoint permits the estimation and search for an optimal cutpoint by confronting the problem as in the spirit of the classical signal detection and as a classification problem. The best model classifier will depend on the dataset under study.So, uses must check for which is the model classifier with the best classification performance for his/her dataset.